Soon, Americans of all ages could be eligible to receive the COVID-19 vaccine.
The University of Virginia’s Dr. William Petri chatted with UVA Today earlier this week as Pfizer asked the Food and Drug Administration to authorize its vaccine for children under 5. Parents could be signing their youngest kids up for shots as early as March, and Petri, vice chair for research in the Department of Medicine, is here to answer several of their potential questions.
Q. In December, progress toward authorization of the vaccine for this age group seemed to hit a snag as clinical trials showed that low doses of the vaccine generated protection in children up to 2 years old, but failed to do so in kids aged 2 to 5. What’s changed since then?
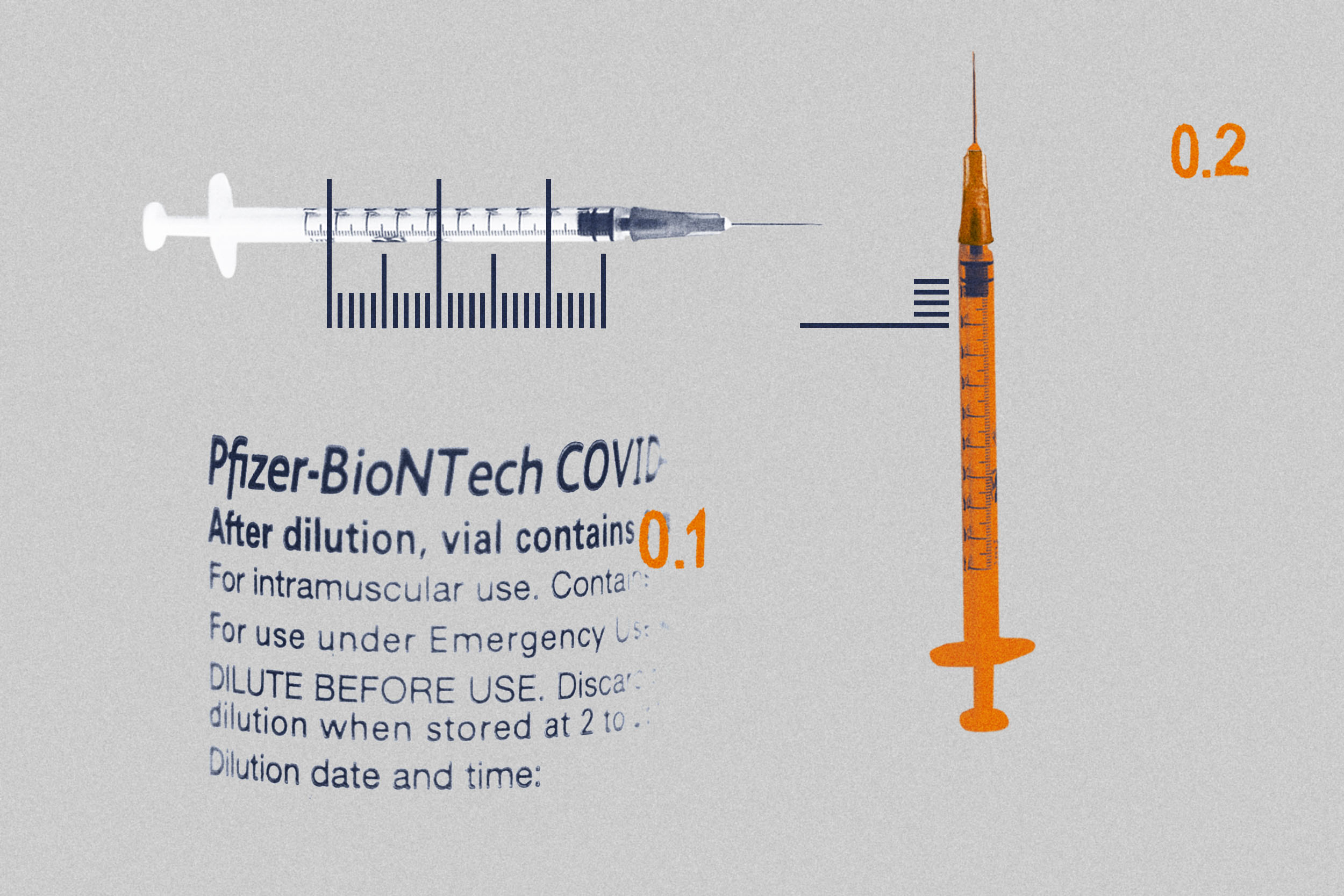
A. You are correct that back on Dec. 17, Pfizer announced in a press release that they were going to change the clinical trial of the Pfizer-BioNTech COVID-19 vaccine in children aged 6 months to 5 years. The clinical trial enrolled 4,500 children in the U.S., Finland, Poland and Spain, with the 6-month to 5-year-old children receiving two 3 µg doses, compared to 10 µg in children aged 5 to 12 years. While the 6- to 24-month-old children had an equivalent immune response to 16- to 25-year-olds (where the vaccine is shown to be highly effective), the 2- to 5-year-olds had an inferior immune response, unfortunately.
This led Pfizer to amend the protocol to add a third dose with the goal to improve the immune response of the 2- to 5-year-olds to that of the 16- to 25-year-olds. This amended clinical trial is not yet completed, so while anticipated that the third dose will improve the immune response, as it has been shown in young adults, this is currently unknown. The clinical trial is expected to be completed in the next few months.

.jpg)