A device that represents a rare advance against glioblastoma – the deadliest form of brain cancer – is now available to newly diagnosed patients after successful testing at the University of Virginia Health System and more than 80 other sites around the world.
The federal Food and Drug Administration has approved the use of the device, called Optune, in newly diagnosed patients after it was shown to extend patients’ survival by approximately three months compared with standard radiation and chemotherapy treatment alone. Optune previously had been approved for use in patients with recurrent glioblastoma, or patients whose glioblastoma had progressed after radiation and chemotherapy.
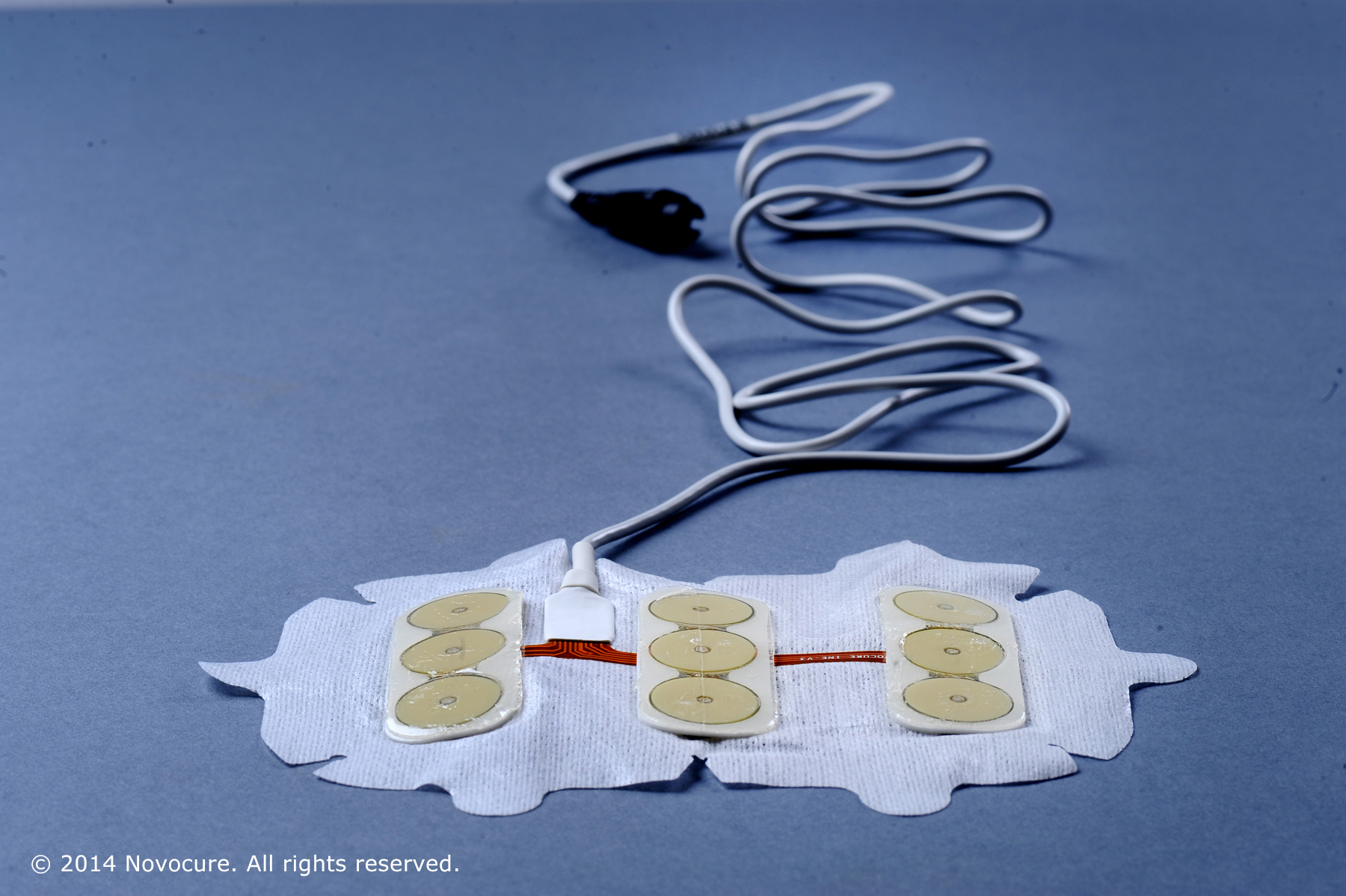
Optune works by emitting low-level, alternating electrical fields through four electrode pads attached to the scalp.
Dr. David Schiff, co-director of the UVA Neuro-Oncology Center, spearheaded UVA’s testing of the device.
“Novel approaches are needed against these very aggressive tumors, and the data from laboratory testing suggested very-low-intensity electrical fields could halt malignant cells from dividing with a favorable safety profile,” he said.
The international clinical trial of the Optune device looked at 695 newly diagnosed patients. It compared the outcomes of those who, following radiation, received standard treatment with the chemotherapy drug temozolomide with the outcomes of patients who received the drug and also wore the device.
Patients who used Optune saw their disease progression halt for an average of about seven months, compared with four months for those receiving only the drug. Overall survival for wearers of the device was 19.4 months after starting treatment, compared with 16.6 months for those who did not wear the device, the FDA reported.
The most commonly reported side effect of the Optune device was skin irritation. However, Optune should not be used by patients who have an implanted medical device, skull defect or sensitivity to conductive hydrogels, such as used during electrocardiograms.
Patients interested in exploring Optune as a treatment option should contact the UVA Neuro-Oncology Center at 434-982-4467.
Media Contact
Article Information
November 12, 2015
/content/new-treatment-deadliest-brain-cancer-available-uva

