Sontheimer said he and his colleagues are hopeful that their research will have a major impact on Alzheimer’s, the always-fatal dementia that affects more than 6 million Americans each year.
“The actual prototype – the one that we’re going to test the behavior with – we should be getting within the next two weeks,” he said. “So we’ve just ramped up, but we’re waiting for the device to be shipped our way. We’re going to start on this before Christmas.”
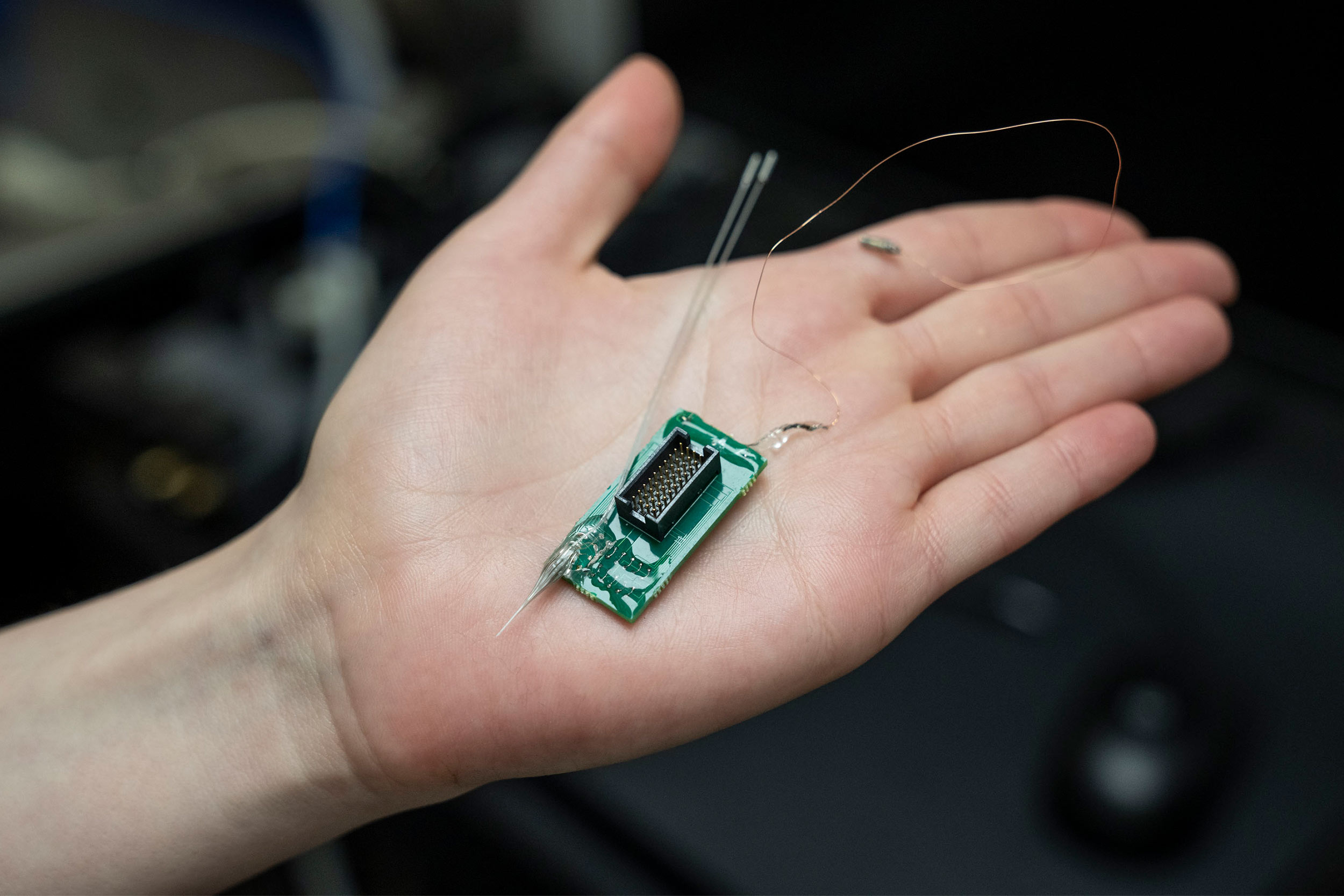
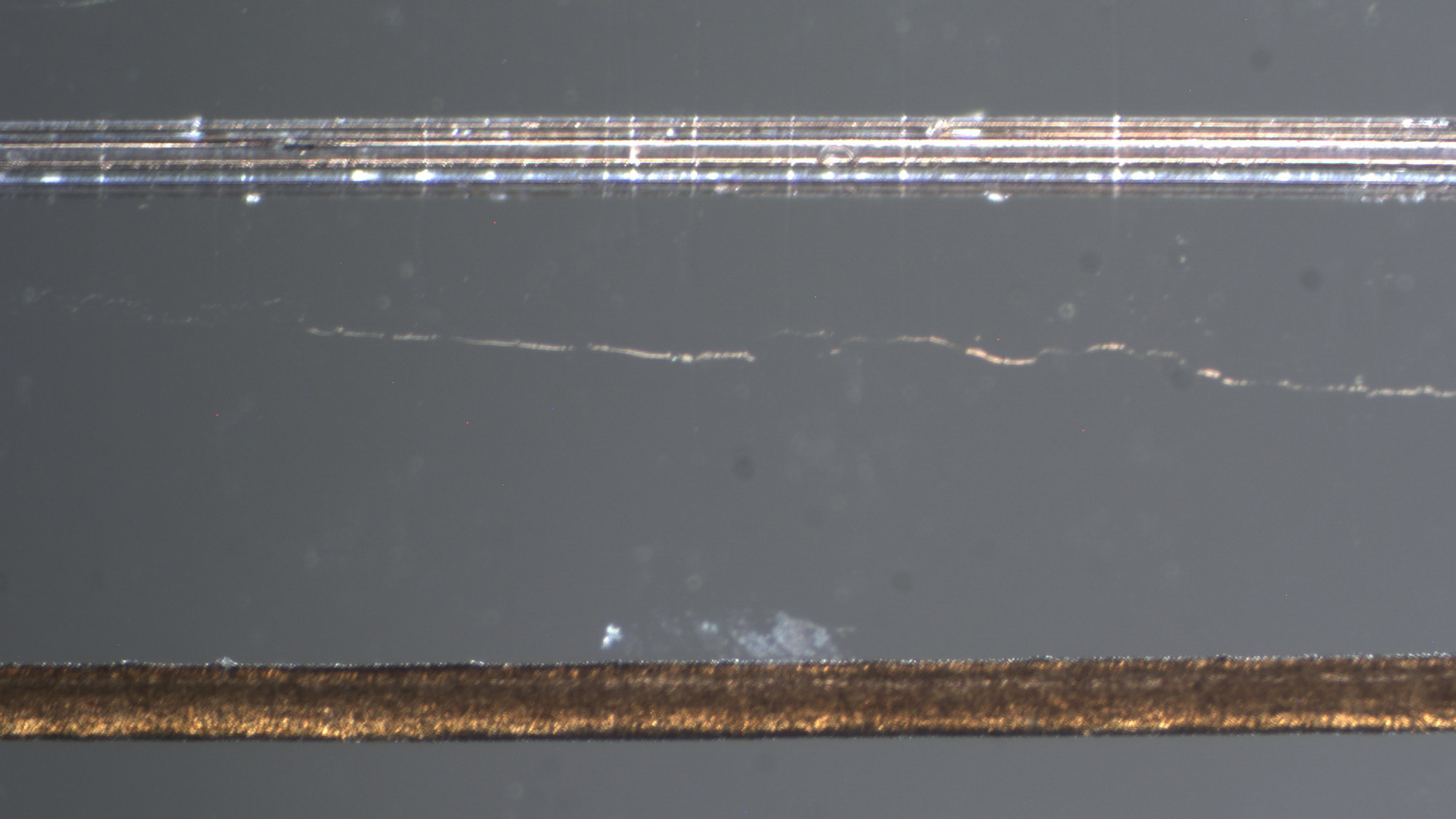
His colleague, Virginia Tech’s Xiaoting Jia, is putting the finishing touches on the fiber-based implant, which is a little smaller than a human hair. Song Hu, of Washington University in St. Louis, created the endoscopic component and will oversee imaging.
The National Institutes of Health awarded the team of Hu, Jia and Sontheimer a one-year grant in November, setting the clock ticking for proof of concept. Should it be successful, they’ll receive funding for another five years to complete the project.
How the Device Will Work
Alzheimer’s starts in the hippocampus, one of the most challenging areas of the brain to image. Because of its depth, current imaging modalities can’t peer inside with great precision.
The researchers’ novel idea was to create a multipurpose device that includes a micro-endoscope that can give a better real-time image of the patient’s situation while also serving as a conduit for therapeutic treatment.
Sitting down with UVA Today staff in his office recently, Sontheimer drew a large circle to indicate a cross section of the device, then smaller circles within, to explain what would be hard to see with the human eye.
In the center is where the endoscope will send light-based signals to get a better view of blood flow and oxygen delivery to the tissue, he said. As the light energy gets absorbed by vessels, it gets converted to heat. That temperature rise then causes a physical expansion that results in the emission of sound waves that are sent back. These sound waves report oxygen tension in the tissue.
Hollow channels along the perimeter of the device will enable drug delivery and contain metallic conductors that will alternately read the brain’s electrical activity and send electrical stimulation to the patient, encouraging blood flow.
The device will extend to the top of the skull, where it will be affixed with dental cement. Ports atop the head will provide access points for each of the channels.
While the nanoscale device may seem ambitious, an earlier version without the endoscope has proved sustainable and effective in interrupting the faulty brain signaling that causes epileptic seizures.
“That’s where pretty much all epilepsy runs, through the hippocampus,” Sontheimer said. “With Alzheimer’s, what we’re envisioning is the real-time monitoring of oxygen, or oxygen tension. The moment oxygen drops, we are immediately administering vasodilators. And it’s sort of a miniaturized approach of what you do when a patient has a heart attack. You give them nitroglycerin to open up all the blood vessels at once and get maximum blood flow back. In this instance, we would maximize blood flow to this region of the brain, hopefully rescuing neural function there.”
If it works, the therapy may also help put an end to a major scientific debate.
Settling a Scientific Dispute
There’s little disagreement about how Alzheimer’s disease starts. Clusters of misfolded proteins form deep within the brain. Known as amyloid plaques, the tangles degenerate nerve cells. As the plaque builds up over time, memory loss occurs.
Patients typically first lose their sense of space, such as where they put the car keys. Eventually, though, they lose a sense of self. Then they lose the ability to maintain autonomic functions like heartbeat and respiration.
But of the many questions that remain about Alzheimer’s, one of them is this: Is the amyloid itself toxic, or do the protein tangles simply cut off blood supply to the nerve cells?
Sontheimer’s lab has previously been able to obtain highly precise images of amyloid encircling and impinging upon vessels, which visually supports his theory.
“We’re hypothesizing that the amyloid actually isn’t directly toxic to neurons as others believe, but that instead, the amyloid builds up around blood vessels, and it strangles them,” Sontheimer said.