October 7, 2010 — HemoSonics LLC, a medical device company founded on technology developed at the University of Virginia, recently secured three federal grants worth nearly $2 million.
The funding came from two highly competitive programs administered by the U.S. Small Business Administration. Small Business Innovation Research, known as SBIR, and Small Business Technology Transfer, known as STTR, seek to further economic development by awarding funding from participating federal agencies to small businesses conducting high-tech research with commercial potential.
Founded in 2004 by U.Va. researchers William F. Walker, Francesco Viola and Michael B. Lawrence, HemoSonics is developing technology to rapidly assess patients' blood for abnormal clotting characteristics. This information could allow physicians to respond more effectively to patients with excessive bleeding or overactive clotting in the operating room, the emergency room and many other clinical settings.
"Most people don't realize that bleeding and blood clots are the main causes of death in the developed world," said Walker, president of HemoSonics and U.Va. professor of biomedical engineering and of electrical and computer engineering. "Heart attack, stroke and pulmonary embolism are all conditions that kill via a blood clot, and studies indicate that pulmonary embolism kills more people than breast cancer.
"Our technology will shed new light on these problems and, we believe, have a notable impact on human health."
HemoSonics' platform technology, sonorheometry, is a novel, ultrasound-based tool developed by the researchers to ascertain specific information about a patient's blood. In determining the time it takes blood to clot, the firmness of a clot and the rates at which a clot forms and dissolves, this technology could enable physicians to identify specific clotting defects and treat them effectively.
The U.Va. Patent Foundation has filed two international patent applications on this technology, which it licensed to HemoSonics for further development and commercialization.
"The research and innovations developed at the University of Virginia are of the highest caliber," said Miette H. Michie, executive director and CEO of the Patent Foundation. "HemoSonics' technology has the potential to have a dramatic, positive impact on patients, and we are proud to be a partner in bringing this technology forward."
Since July, HemoSonics has been awarded nearly $2 million in federal funding through the SBIR and STTR programs, including $1.6 million from the National Institute of Diabetes and Digestive and Kidney Diseases; $298,000 from the National Heart, Lung and Blood Institute; and $70,000 from the Office of Naval Research.
Thomas C. Skalak, U.Va. vice president for research, said such major federal awards are critical to new technology-based ventures. "We are very proud to see this U.Va. research being moved to the marketplace, where it will have an impact on people's health and potentially create new jobs," he said.
He added, "The accelerated pace of HemoSonics' growth is a tribute to the vision and persistence of its technical leaders: Bill Walker, Francesco Viola and Mike Lawrence."
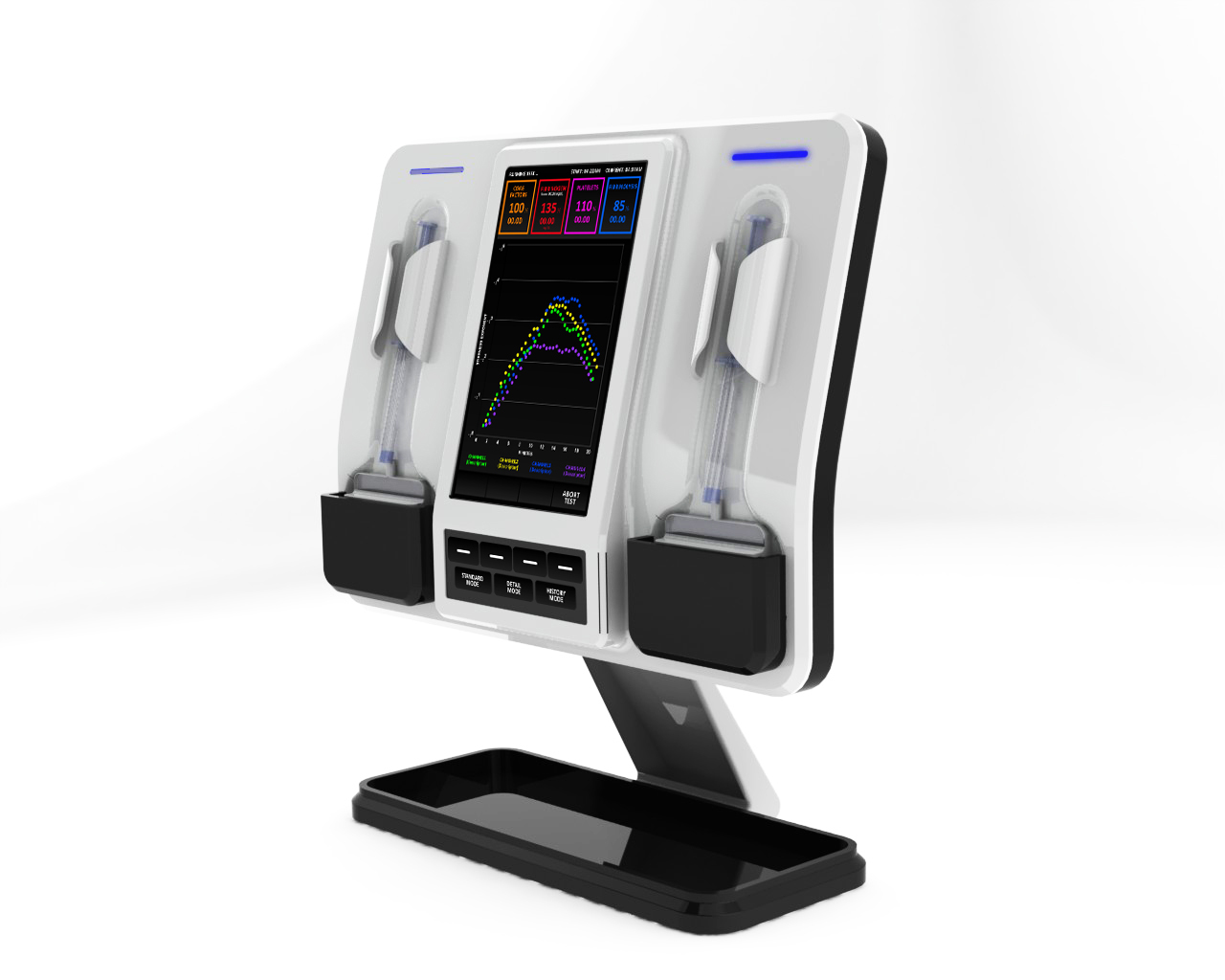
With its new funding, HemoSonics plans to develop a point-of-care diagnostic device called the Global Hemostasis Analyzer, which will bring the technology to the patient's bedside and seek to eliminate the guesswork associated with treating bleeding conditions.
"In patient care settings, bleeding patients are often treated by blind administration of blood products," said Viola, HemoSonics' vice president of engineering and technology and research assistant professor of biomedical engineering at U.Va. "This process is clearly inefficient, often resulting in wasted resources, increased costs and even harmful consequences for the patient.
"Our initial product will quickly provide the necessary information to guide transfusion, therefore saving lives, money and resources," added Viola, who is the principal investigator of the grants.
This technology has also been funded by the Wallace H. Coulter Translational Research Partnership, the National Institutes of Health and angel investors.
Skalak said, "The researchers' original work inside of the University was a team effort that exemplifies the U.Va. translational research partnership with the Wallace H. Coulter Foundation, which is committed to realizing the dream of science serving humanity."
The funding came from two highly competitive programs administered by the U.S. Small Business Administration. Small Business Innovation Research, known as SBIR, and Small Business Technology Transfer, known as STTR, seek to further economic development by awarding funding from participating federal agencies to small businesses conducting high-tech research with commercial potential.
Founded in 2004 by U.Va. researchers William F. Walker, Francesco Viola and Michael B. Lawrence, HemoSonics is developing technology to rapidly assess patients' blood for abnormal clotting characteristics. This information could allow physicians to respond more effectively to patients with excessive bleeding or overactive clotting in the operating room, the emergency room and many other clinical settings.
"Most people don't realize that bleeding and blood clots are the main causes of death in the developed world," said Walker, president of HemoSonics and U.Va. professor of biomedical engineering and of electrical and computer engineering. "Heart attack, stroke and pulmonary embolism are all conditions that kill via a blood clot, and studies indicate that pulmonary embolism kills more people than breast cancer.
"Our technology will shed new light on these problems and, we believe, have a notable impact on human health."
HemoSonics' platform technology, sonorheometry, is a novel, ultrasound-based tool developed by the researchers to ascertain specific information about a patient's blood. In determining the time it takes blood to clot, the firmness of a clot and the rates at which a clot forms and dissolves, this technology could enable physicians to identify specific clotting defects and treat them effectively.
The U.Va. Patent Foundation has filed two international patent applications on this technology, which it licensed to HemoSonics for further development and commercialization.
"The research and innovations developed at the University of Virginia are of the highest caliber," said Miette H. Michie, executive director and CEO of the Patent Foundation. "HemoSonics' technology has the potential to have a dramatic, positive impact on patients, and we are proud to be a partner in bringing this technology forward."
Since July, HemoSonics has been awarded nearly $2 million in federal funding through the SBIR and STTR programs, including $1.6 million from the National Institute of Diabetes and Digestive and Kidney Diseases; $298,000 from the National Heart, Lung and Blood Institute; and $70,000 from the Office of Naval Research.
Thomas C. Skalak, U.Va. vice president for research, said such major federal awards are critical to new technology-based ventures. "We are very proud to see this U.Va. research being moved to the marketplace, where it will have an impact on people's health and potentially create new jobs," he said.
He added, "The accelerated pace of HemoSonics' growth is a tribute to the vision and persistence of its technical leaders: Bill Walker, Francesco Viola and Mike Lawrence."
With its new funding, HemoSonics plans to develop a point-of-care diagnostic device called the Global Hemostasis Analyzer, which will bring the technology to the patient's bedside and seek to eliminate the guesswork associated with treating bleeding conditions.
"In patient care settings, bleeding patients are often treated by blind administration of blood products," said Viola, HemoSonics' vice president of engineering and technology and research assistant professor of biomedical engineering at U.Va. "This process is clearly inefficient, often resulting in wasted resources, increased costs and even harmful consequences for the patient.
"Our initial product will quickly provide the necessary information to guide transfusion, therefore saving lives, money and resources," added Viola, who is the principal investigator of the grants.
This technology has also been funded by the Wallace H. Coulter Translational Research Partnership, the National Institutes of Health and angel investors.
Skalak said, "The researchers' original work inside of the University was a team effort that exemplifies the U.Va. translational research partnership with the Wallace H. Coulter Foundation, which is committed to realizing the dream of science serving humanity."
Media Contact
Article Information
October 7, 2010
/content/uva-start-hemosonics-secures-2m-federal-funding